Abstract
Background: The mechanisms that cause the progression of myelodysplastic syndrome (MDS) are poorly understood. Little is known about major signaling networks and energy metabolism in MDS cells as patients progress from low risk (LR) to high risk (HR) disease and from high risk to secondary acute myelogenous leukemia (sAML). As many as 30% of HR MDS patients progress to sAML and a portion of LR MDS patients progress to HR. The goal of this project is preventing progression by identifying MDS-specific targets for therapy. A deeper understanding of the metabolic properties of leukemia stem cells (LSCs) in AML has shown these cells are uniquely vulnerable to venetoclax and azacitidine (Ven/Aza) (Pollyea et al, Nat. Medicine, 2018) and metabolic changes cause resistance to Ven/Aza (Stevens et al, Nat. Cancer, 2021). Little however is known about the contribution of metabolism to the pathogenesis of MDS. The contributing factors to progression including metabolic properties, transcriptional programs, and immunophenotype are examined in this study.
Methods: Bone marrow specimens from MDS patients at various disease stages, including serial samples during progression, were obtained. Single cell techniques including mass cytometry, antibody based single cell RNA sequencing (CITE-Seq) and transcriptional profiling with RNA sequencing were used to elucidate novel mechanisms of progression. Selective targeting of primitive MDS cells was tested using several agents.
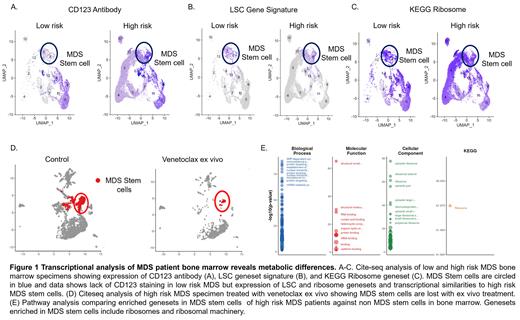
Results: Our previous work characterizing MDS stem cells (MDSC) showed significant similarities between MDSCs and AML LSCs (Stevens et al, Nat. Communications, 2018). However, little is known about lower risk disease. In order to understand transcriptional changes and their relationship to metabolism across pathogenesis, the transcriptome of blasts from patients with LR, intermediate (INT), or HR IPSS scores was investigated. The first major transcriptional difference identified was enrichment of glycolysis pathway at LR and INT stage. In contrast, HR MDS demonstrated enrichment of oxidative phosphorylation. Furthermore, comparison of intermediate to HR MDS showed increased RNA polymerase and Ribosome pathways at the HR stage. These changes demonstrate the progressive alteration of metabolic properties during MDS pathogenesis with cells first relying on mechanisms associated with normal stem cells (i.e. glycolysis) and later transitioning to a state associated with AML stem cells (i.e. reliance on oxidative phosphorylation).
Using serial specimens of patients of who progressed from LR to HR MDS we performed CITE-Seq and mass cytometry. CITE-seq in serial specimens showed up-regulation of protein translation and oxidative phosphorylation in a subset of MDS stem and progenitor cells (CD34+ at transcript and antibody level) present at LR stage and conserved at HR stage (Fig 1A-C). MDSCs also acquired surface antigens including CD99 and CD52 upon progression from LR to HR. Analysis of the mass cytometry data showed significant overlap with CITE-Seq data including increased CD123+ and MCL1 expression in MDS stem cells upon progression.
In order to understand therapeutic vulnerabilities as they relate to progression, we investigated ex vivo drug response in LR and HR specimens. MDS samples were challenged with two regimens, Ven/Aza, a regimen known to inhibit OXPHOS; and omacetaxine and azacitidine (Oma/Aza), which inhibits translation. CITE-seq showed that MDSC were selectively sensitive to these agents (Fig 1D). Importantly, addition of either drug regimen caused ablation of MDSC at LR and HR stages and these changes were most profound in cells with LSC properties.
Based on preclinical findings, we are investigating MDS patients treated with Ven/Aza or Oma/Aza via CITE-seq and metabolomics for correlation of clinical response with properties of MDSC. Preliminary studies show that patients that respond to Oma/Aza present with a population of MDSC with transcriptional signatures of protein translation and LSCs (Fig. 1E). Studies are underway to investigate overlapping properties of ven/aza resistance in AML to resistance in MDS specifically investigating fatty acid metabolism in MDSC.
Conclusions: Analysis of MDS patient bone marrow reveals acquisition of aberrant metabolic properties at both low and high risk stages of disease. These distinct aspects of MDSC biology create unique and targetable features.
Pollyea: Genentech: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy; Syndax: Consultancy; Takeda: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Consultancy; Amgen: Consultancy; AbbVie: Consultancy, Research Funding; Agios: Consultancy; Glycomimetics: Other.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal